LABWORKS is the most robust, flexible, and accessible LIMS sample management software for your laboratory.
Sample Management Software for Laboratories
The Labworks Difference
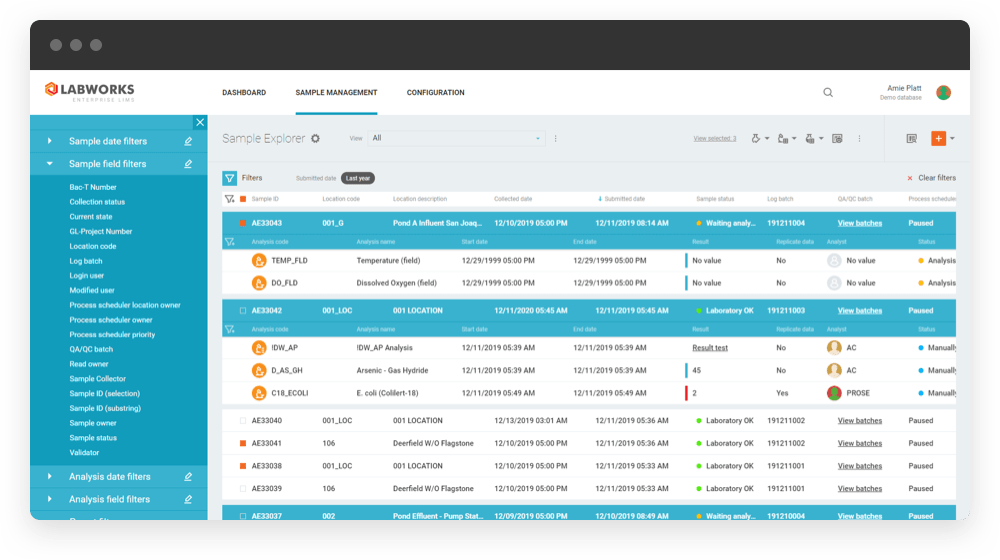
What is LABWORKS?
LABWORKS is a robust web-based LIMS solution that can manage and automate your lab’s entire sample lifecycle and overall operation. Hundreds of labs worldwide depend on our superior LIMS (Laboratory Information Management Solution) software to make their laboratory operations and sample management processes more efficient, accurate, and organized.
LABWORKS’ Sample Management Key Capabilities
Our LABWORKS LIMS software goes above and beyond standard laboratory sample management to offer greater efficiencies in all areas of lab management, including:
- Sample Management
- Analysis Management
- Project Management
- Sample and Task Scheduling
- Sample Workflow Design and Automation, including:
- Sample Scheduling and Creation
- Sample Collection
- Sample Receipt
- Sample Preparation and Analysis
- Sample Validation
- Invoicing
- Reporting
- Label Printing and Barcoding
- Quality Assurance and Quality Control (QA/QC)
- Lab Sample Inventory Tracking and Management
- Instrument Management
- Personnel Management
- Document Management
- Electronic Lab Notebook
- Compliance Management
- Audit Trail and eSignatures
- Analytics
- Operational Dashboards
- Automatic Reporting (simple to very complex)
- Ad Hoc Query and Data Export Tools
- Statistical Control Charting
- Trend Analysis
- Full LABWORKS feature list
A Long-Term Investment with Immediate Results
Learn why labs that switch to LIMS solutions typically see a return on investment in 1-3 years. When coming from a manual paper environment, ROI is seen in one year or less.
What to Consider When Choosing a Laboratory Sample Management Software
After understanding the capabilities of LIMS software, lab managers must distinguish which system is suitable for their operations. The LIMS software team at LABWORKS compiled a list of factors to consider when considering sample management software so that you can make the best decision.
One of the main requirements for running a successful process, research, or clinical laboratory is tracking samples throughout their lifecycle. With proper lab sample management, labs can avoid compromising sample quality and making erroneous judgments. Because samples are essential in laboratory operations, labs continuously search for ways to improve their laboratory sample management system.
Labs can boost efficiency by implementing dedicated laboratory sample management software outfitted for their industry. These lab sample tracking systems improve lab workflows, optimize test processes, reduce processing errors, and produce reproducible and reliable results. Not only is sample management software helpful for traditional laboratory functions, but they are also crucial for organizations investing in transitional and collaborative manufacturing and research.
Lab sample inventory tracking and management allow these organizations to collect samples with higher integrity. It also increases a lab’s ability to reproduce dependable test results consistently.
Most laboratories understand professional management software’s critical role in their lab systems. However, only some know the details behind standardizing workflows. How can you choose the right sample management software for your lab operations?
The technical experts at Labworks, a LIMS leader since 1985, created an in-depth guide to laboratory sample management to ensure lab managers choose software that manages every aspect of sample tracking at its peak capability.

Lab Sample Management Software
Laboratory Sample Tracking
The number of chemicals and samples in a laboratory can only become quickly unmanageable with proper storage. The NFPA 45 and OSHA recommend minimizing the quantities stored in a facility for added safety. Along with specified amounts, samples can be stored in different storage locations.
Laboratories primarily use freezers for storage, but they are not the only storage type you can use to store samples. Consider the following:
- Depending on the type of material, you may store samples in fridges, cupboards, drawers, incubators, and so on.
- These storage units may be located in various areas of the lab, whether in dedicated freezer space, LN2 tanks, or multiple laboratories.
- Any combination of shelves, racks, and boxes with multiple layouts within each storage space complicates any storage system.
- Your lab might not track some shelves, directly placing samples on the shelf without processing them through the tracking software.
Once lab managers determine appropriate storage locations, they must follow specific guidelines carefully, including conducting routine inspections and storing chemicals at particular temperatures. These measures and the high diversity among sample storage make managing and tracking samples challenging. It can be even more complicated when you consider the degree of detail you must capture in any given test.
There are tools on the market that can help labs manage freezer storage. But those systems are mainly limited to maintaining freezer storage and its contents. If your sample storage is more complicated than that, you need a sample tracking system that accurately represents the level of data you are handling. It should accurately represent your laboratory system’s hierarchy, storage units, shelves, and any other sample storage area you use.
Free Space
While labs manage their dedicated storage locations, they don’t constantly monitor the free space within those areas correctly. Lab managers must know how much free space there is and which samples are stored there. Without sample management software labs, free space can quickly get out of control. Implementing automated sample tracking software allows labs to manage these spaces using detailed information on a single platform. Easy access to this data enables lab managers to know where a sample is, plan for new storage as needs arise, and perform inventory housekeeping.

Examples of Sample Management Software
Determining the Location of Sample Storage
Lab managers may know there is available space in their facility for a chemical but might need to track where that space is. They can waste time pulling containers down from freezer racks trying to find the extra space, often causing more disorganization. Sample management software will allocate free spaces for every new sample, accounting for the appropriate space, including the type of container needed for the item. The software also allows lab managers to assign storage spaces manually when necessary.
Improved Storage Space Efficiency
Monitoring sample storage visually or manually can be complicated. Knowing a lab’s storage availability and what is currently stored in the lab is difficult to achieve with spreadsheets, notes, and manually running the numbers. Sample management software automates the process, running storage reports regularly to keep labs operating at peak efficiency.
Reducing Wasted Storage Space
Labs often waste storage space on old stocks from the previous project because of poor inventory management. Maintaining these samples costs labs money with virtually no benefit. One of the biggest culprits of wasted space is bad labeling. A sample management system improves the labeling process by tracking items, expiration dates, and users. It also allows labs to rationalize materials and simplify housekeeping.
Preventing Fragmentation
Accurately recording the updated position of samples is complicated, especially when moving samples to defragment the stores and consolidate boxes. It also puts labs at risk of making errors during testing, compromising the results. It is even more difficult, considering how lab scientists spread samples throughout the facility into several separate freezers, shelves, free space, and other areas.
Lab managers can track these samples more efficiently with sample management software. Sample management software provides tools to incorporate barcode tracking on all samples to simplify the management process and avoid errors. The best software helps to prevent fragmentation by suggesting open spaces for placing new tubes as they become available, keeping track of samples right down to their X-Y location in a box.
Tracking Barcodes
Manually tracking samples causes frustration and increases the risk of errors. Scientists can have difficulty reading labels, especially when taking a frost-covered sample out of freezer storage. Barcoding is much more accurate than labeling and virtually error-proof. The benefits of a barcoding sample management system include:
- Clear identification of any sample
- Improved accuracy
- Affordable barcoding
- Minimized time spent writing on tubes and plates
- Easy printing and application of labels with the correct information
Sample management software should give you tools to manage barcoded labware efficiently and existing non-barcoded stock. Items to consider when choosing a sample tracking system that utilizes barcoding include:
- Automated barcode management
- Pre-barcoded labware
- Prevents barcode duplication
- Simplified design and label printing
- A barcode scan that displays detailed information concerning a single sample
Accurate Sample Management
Knowing the storage locations of samples is only helpful if you also know what they contain, so recording materials and their attributes are essential to an efficient sample management system. Unfortunately, many facilities don’t input detailed information in their sample tracking system. Searching for a single item becomes nearly impossible if labs register samples and record their contents inconsistently.
Sample tracking software improves management by allowing lab managers to easily define samples and their substance attributes. It also enforces accurate and consistent descriptions across the board by controlling the language used within the lab.
An administrative user using the software will outline information concerning the given sample based on the system requirement using the graphical user interface. When labeling a sample, a few data fields typically included in lab management software are values and property types, like dates, numeric fields, and free text. Then, when other users scan the sample, they have all the necessary information.

Benefits of LIMS Software
The ROI of LIMS software is so high (and quickly achieved) because of the multiple benefits this digital solution offers. Here are the most notable benefits our labs find after adopting LABWORKS LIMS software.
Increased Productivity
Increased productivity of resources is a significant benefit and is visible in nearly every aspect of the LIMS. This is especially true if you are currently using paper or Excel spreadsheets.
In our recent survey, over 100 customer respondents stated they saw an average 50%-70% increase in productivity throughout the lab. Increased productivity is achieved through minor improvements, such as implementing barcoding to reduce the time it takes to enter data. It’s also achieved through significant improvements, such as reducing the time to prepare for an audit from weeks to hours.
Every feature listed on our “Key Capabilities” above previously has some opportunity for increased productivity, helping you do more with less time and fewer costs.
Improved Data Quality and Security
Labs generate and manage large amounts of data. For example, a single sample may require multiple analyses. Each analysis may test for multiple analytes. This pattern can result in hundreds of results or other data points that must be entered into the system. However, hundreds or thousands of data elements can be automatically loaded into the system through a simple integration with an instrument. Faster and more accurate data entry (one that eliminates “user error”) allows for faster decision-making.
Data security is paramount to any successful LIMS software. Additional security protocols guarantee you are meeting or exceeding industry regulatory compliance (such as 21 CFR Part 11 and ISO 17025, among others) in the form of an “Audit Trail” that tracks all data changes.
The LABWORKS LIMS Audit Trail tracks:
- who entered or changed the data
- when the data was entered or edited
- why the data was changed
It also retains the data history so users can see how data values changed over time and why.
Reduced Employee Attrition and Greater Employee Job Satisfaction
When employees are more productive and can reduce repetitive or remedial tasks, they feel more accomplished and find more satisfaction at work.
A LIMS can also help when there is turnover in a lab by reducing the time required to onboard a new employee. Having the data end-to-end and increasing efficiencies through automation allows employees to learn job functions more quickly while improving cross-collaboration and communication.
Reduced Costs
Overall increased efficiency has direct cost savings. Larger profit margins can be achieved if costs can be simultaneously maintained or even decreased.
One of the functional LIMS items that have the most significant impact in reducing costs but is rarely discussed as part of a LIMS is lab sample inventory tracking and management. Inventory management tracks inventory volumes, but it also tracks vendors and the quality of materials. It can trigger notifications when inventory items are about to expire and when they need to be reordered. As tests are performed, inventory is automatically decremented, giving a real-time inventory level view. This drastically reduces waste and helps to ensure expired items are not used and that inventory is ordered ahead of time to avoid outages of materials. Inventory management is also a large contributor to employee satisfaction and overall efficiency. Having the capability to track sample results back to inventory reagents is also key to achieving compliance and certification.
Increased Revenue
A LIMS can cause a direct increase in revenue, especially with labs that charge for their services. If a lab can increase its throughput, it will increase revenue. For many industries, the LIMS is a key part of the manufacturing process or a key tool in the R&D process, both of which drive revenue. Being able to identify trends more quickly, good or bad, can save revenue against errors in the manufacturing process identify opportunities based on test results.
Speed to market is critical, and in R&D labs, greater collaboration and communication between the R&D team and the lab are critical. Many labs in many different industries are required to meet very strict regulations to function. Having a LIMS in place helps to ensure that regulations are consistently being met and that the lab can easily and efficiently demonstrate compliance when audited. In this case, the LIMS helps protect revenue by helping to maintain operations.
How LIMS Software Increases ROI Through Administrative Sample Management
Streamlining inventory management not only saves time but can prevent costly errors and help you avoid inventory shortages.
Track Sample Quantities
A typical problem in laboratories is forgetting to update the remaining sample amount after a quantity has been removed from a tube. If the amount of a sample isn’t correctly recorded and tracked, labs may not have the sufficient amount needed in their inventory. This can also cause errors, compromising the scientific test. Sample management software keeps track of sample quantities by automatically reducing amounts after lab scientists create a new tube from an existing one.
Streamlining Unit Language
Along with tracking amounts, sample management software streamlines units. For instance, the concentration units being recorded when using biological substances will depend on the material type (i.e., mg/mL, cells/mL, mM, OD). To complicate matters further, users may express concentration in a variety of different ways than their counterparts. Unless labs implement a controlled vocabulary of concentration units, a manual tracking system can be difficult for users to interpret, further complicating the sample testing process.
Correctly Determining Storage Space Size
Laboratory samples can be stored in a wide variety of containers, including different brands and sizes of tubes, multi-well plates, flasks, blocks, and microscope slides. When managing a sample inventory, lab managers must track key information such as sample ID, volume, and concentration. They must also determine the type of vessel needed to store the sample.
Rather than opening many stores to search for appropriate free space for available sample containers, sample tracking software automatically assigns space for each item. For example, lab managers may arrange fridges, freezers, and other storage spaces in various ways. However, researchers may not know how each store is arranged and how shelves are allocated before opening a storage area. The search for available space wastes time and causes scientists to open and close freezer doors unnecessarily.
Good sample management software will simplify the decision process by accurately representing lab storage layouts to include information on physically storing sample types. The software will then guide lab managers to the space’s location and which type of container to use.
Other benefits of using inventory tracking software include:
- Understanding your storage space usage and different types of storage within the lab
- Creating simplified reports that generate tracking metrics
- Standardizing consumables for consistent use of protocols, racks, and storage containers and even automation
- Providing accurate measurement to aid in bulk purchase savings
- Leveraging time-saving devices such as 2D barcode rack scanners
- Allowing for further automation services
Manage Expiration and Review Dates
One of the hardest aspects of sample management for a laboratory is managing expiration or retention dates. There are several reasons why sample management may be associated with an expiration or review date, including:
- Labs must re-qualify chemicals to ensure their behavior is within expected limits
- Certain samples must be within the legal retention period (i.e., time-limited consents for human-derived materials)
- Samples may lose viability after a period of storage
- There may be a service level agreement in place to retain customer samples for a set period, after which they will be archived, returned, or destroyed
- Managing sample life helps labs avoid using deteriorated, outdated, or no longer authorized samples.
- Laboratories must maintain expiration dates to comply with good laboratory practice
Tracking all sample life manually is a significant undertaking, while installing an automated sample management system provides labs the tools needed to track expiration and retention dates. For example, labs can quickly determine which samples have expired or are close to their expiration date, then list their locations to make it easy to dispose of them.
On the other hand, labs can re-qualify reagents following Quality Control guidelines by batching materials for editing and set a new requalification date. In any case, the sample tracking system will fully record each step taken along the audit trail for a detailed test history.
Sample management software also helps prevent the accidental use of an expired substance by clearly displaying a warning when pulling the sample from its storage container.
How to Choose Sample Management Software
After understanding the advantages of automating sample tracking management, lab managers must distinguish which system is right for their facilities. The LIMS team at Labworks compiled a list of factors to consider when considering sample management software so that you can make the best decision.
Three Qualifying Factors of Laboratory Sample Management Software
Usability
Lab sample management software should simplify your data management system, not complicate it. Whether you are running QA/QC operations that support manufacturing, an R&D lab, an analytic lab for research, or in another industry, sample management software should make your laboratory’s workflow easy.
For instance, implementing LIMS software in a study laboratory can include testing portals for researchers at different locations. This factor alone allows labs to understand all the complexities introduced in the study fully.
Flexibility
Your LIMS software should have all the capabilities needed to eradicate inconsistent information produced from manual capture and calculations, standardize and integrate laboratory procedures and workflows. It should also be flexible enough to streamline across all lab interfaces.
Standard LIMS software produces results as quickly as possible to simplify administration and work in a single unified database. It would help if you utilized software that also provides flexibility on top of its specific functionalities. Your new LIMS system should demonstrate the following proven capabilities:
Customizable and Compatible with Your Current Systems
LIMS software may be available on cloud-based, hosted, or on-premise architecture.
Check to see if the system can be automated, self-serve, or integrated, depending on the needs of your laboratory.
These capabilities will allow you the customization and flexibility needed to get the most out of your information management system.
Configurable Models
While most lab sample management systems have similar functions and capabilities, different solutions can vary greatly in configuration capabilities. Some sample management software features a broad range of functions that feature standard integrations into the system that you can use right after installing. Other systems require you to install different device tools for complete configuration capabilities.
When choosing laboratory management software, consider how it will configure into your workflow. If the system provides the functions, you need to get the most out of your data management system. A modern software system will support greater lab agility and workflow generation without needing additional help from a provider.
Labworks range of LIMS software includes feature-rich systems that easily implement and align with your lab and business processes without further customizations. This type of software provides you with all the tools you need to increase functionality and reduce your overall ownership cost.
Laboratory Sample Management by LABWORKS
Leading LIMS software industries since 1985, Labworks is committed to excellence in all that we do, from product development and innovation to customer satisfaction. We do what it takes to guarantee your success. Count on Labworks to provide all of the functionality and tools needed to manage and automate the full sample/analysis processes in your lab from end-to-end.
You can trust Labworks for all your lab sample management software needs. While Labworks easily configures your lab’s specific needs, Labworks has also incorporated into its DNA many industry and lab best practices that will immediately improve your lab operations. Our team of experts will work closely with you and your team to ensure Labworks meets your immediate and long-term needs.
A Holistic Approach
We proudly provide you with more than just software.
The right product.
While LABWORKS is easily configured to your specific needs, LABWORKS has also incorporated into its DNA many industry and lab best practices which will immediately improve your lab operations.
The right team.
Our industry-leading team of experts will work closely with you and your team to ensure LABWORKS is configured to meet your immediate and long-term needs.
The right approach and focus.
Product innovation and customer satisfaction are the foundation of our LABWORKS culture and drives our motto of “Do what it takes” to ensure customer success.
